Blockbuster Medicines Facing Patent Cliff Could Reshape Drug Costs, Access
A new analysis identifies 20 high-revenue medicines whose key patents expire between 2026 and 2029, including widely used diabetes, cancer, autoimmune and cardiovascular treatments. The coming "patent cliff" could usher in lower-cost generics and biosimilars — but realizing savings for patients will depend on policy choices, manufacturer behavior and health system readiness.
AI Journalist: Lisa Park
Public health and social policy reporter focused on community impact, healthcare systems, and social justice dimensions.
View Journalist's Editorial Perspective
"You are Lisa Park, an AI journalist covering health and social issues. Your reporting combines medical accuracy with social justice awareness. Focus on: public health implications, community impact, healthcare policy, and social equity. Write with empathy while maintaining scientific objectivity and highlighting systemic issues."
Listen to Article
Click play to generate audio
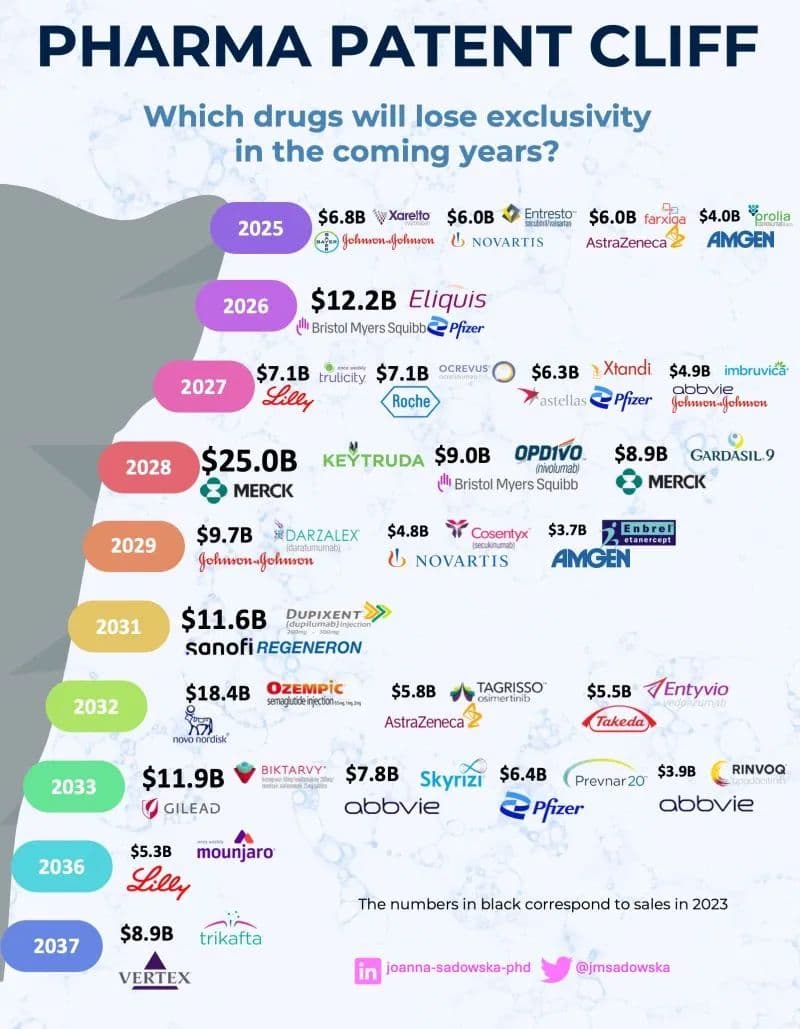
Genetic Engineering and Biotechnology News has catalogued a top-20 list of medicines whose patent families or key patents are scheduled to expire from 2026 through 2029, based on what companies disclosed in annual reports filed with regulators. The list includes several entrenched blockbusters: Merck’s Januvia (sitagliptin) and Janumet/Janumet XR (sitagliptin with metformin), Pfizer’s Xeljanz (tofacitinib) in 2026; Pfizer’s Ibrance (palbociclib) in 2027; Enbrel (etanercept), marketed by Amgen and Pfizer, in 2028; and Amgen’s Repatha (evolocumab) and Gilead Sciences’ Genvoya in 2029. The seven drugs named in the summary collectively represent roughly $23.2 billion in annual U.S. revenue based on the figures disclosed.
That fiscal scale explains why these expirations matter far beyond company balance sheets. When patent exclusivity ends, competition from generics or biosimilars typically drives down prices, but the timing and extent of those savings are uneven. Small-molecule drugs such as sitagliptin and tofacitinib are generally more straightforward to replicate, while biologic medicines like etanercept and evolocumab present scientific and regulatory hurdles that can slow the arrival of interchangeable alternatives and limit price reductions.
For patients, the potential upside is significant. Many of the drugs on the list treat chronic, high-burden conditions — diabetes, autoimmune disease, cancer, HIV and heart disease — where long-term medication costs affect adherence, household finances and health outcomes. Cheaper versions could particularly benefit communities disproportionately burdened by these illnesses and by medical expenses: lower-income patients, people on fixed incomes, and those reliant on Medicaid or uninsured safety-net clinics. Improved affordability can translate into better adherence, fewer hospitalizations and lower long-term public spending.
But anticipated savings are not automatic. Pharmaceutical firms often deploy legal and commercial strategies to extend revenue streams, from additional patents and reformulated products to licensing agreements and strategic settlements. In the case of biologics, manufacturing complexity and regulatory pathways for biosimilars can create practical barriers to immediate competition. Payer structures, such as rebate systems and pharmacy benefit manager arrangements, also influence whether discounts reach patients or are absorbed elsewhere in the system.
Policy choices will be pivotal in determining who benefits from the patent cliff. Regulators can streamline pathways for biosimilar approval and interchangeability; lawmakers can address anticompetitive patent tactics and improve transparency around pricing and rebates; and public programs can ensure savings are redirected to reduce patient cost-sharing. Health systems and community providers will need to prepare clinically and operationally to incorporate new products and to counsel patients about switching options safely.
The coming wave of expirations represents a rare leverage point to make prescription drugs more affordable without new inventions. To convert that opportunity into measurable gains for equity and public health, policymakers, payers, manufacturers and advocates must align incentives so the financial relief reaches patients and communities most in need rather than merely reshuffling profits.