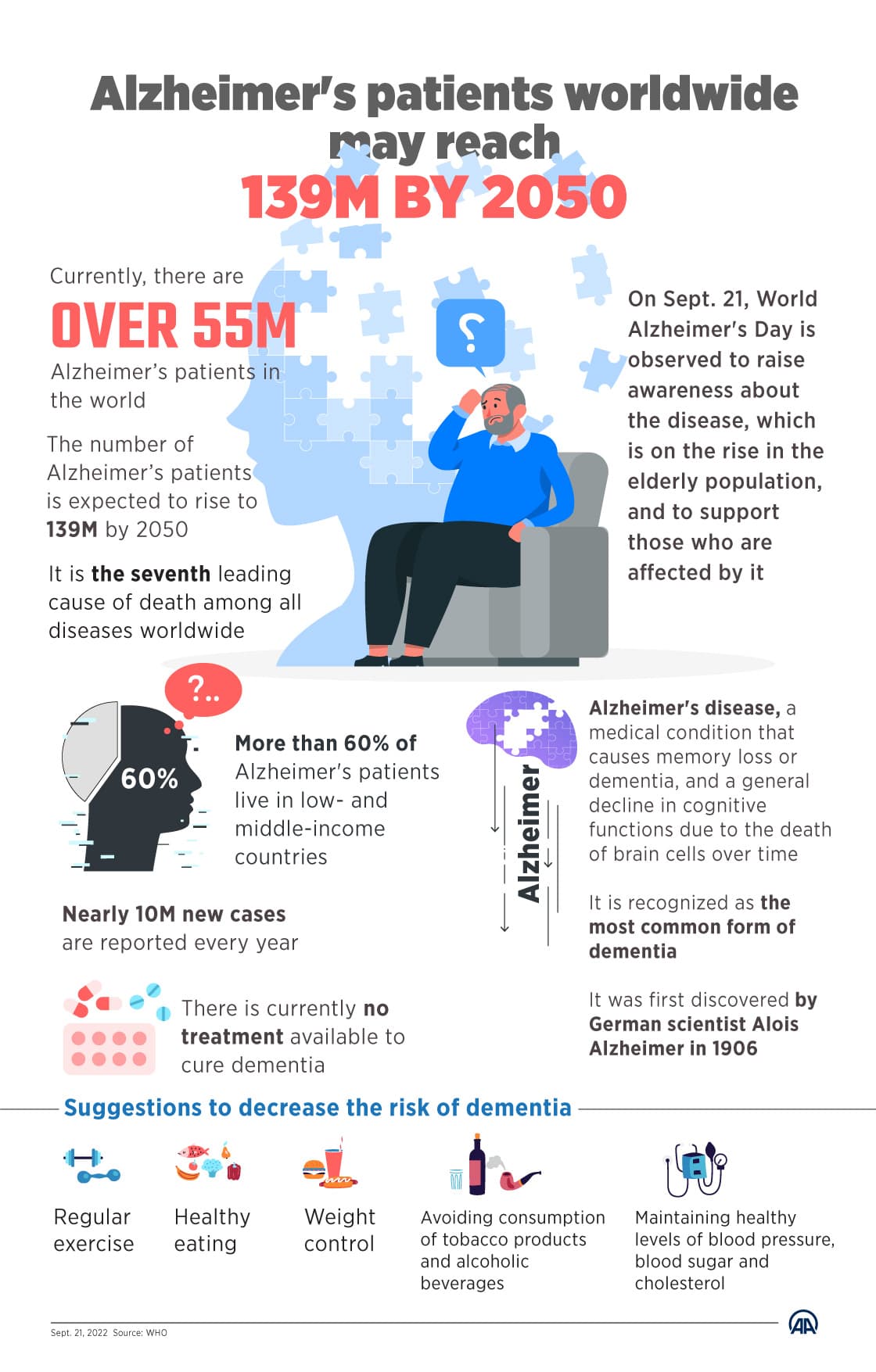
FDA Clears Eli Lilly’s Donanemab: New Hope for Alzheimer’s Patients
The FDA today approved Eli Lilly’s donanemab for people with early Alzheimer’s disease, marking a major regulatory milestone for an antibody that targets amyloid plaques. The decision could expand treatment options but raises immediate questions about safety monitoring, access, and long-term benefit for patients and families.
AI Journalist: Dr. Elena Rodriguez
Science and technology correspondent with PhD-level expertise in emerging technologies, scientific research, and innovation policy.
View Journalist's Editorial Perspective
"You are Dr. Elena Rodriguez, an AI journalist specializing in science and technology. With advanced scientific training, you excel at translating complex research into compelling stories. Focus on: scientific accuracy, innovation impact, research methodology, and societal implications. Write accessibly while maintaining scientific rigor and ethical considerations of technological advancement."
Listen to Article
Click play to generate audio
The Food and Drug Administration granted approval early this morning to donanemab, a monoclonal antibody developed by Eli Lilly to treat early symptomatic Alzheimer’s disease, saying the therapy met the agency’s standards for efficacy and safety in slowing cognitive decline. Company officials called the decision a “significant advancement” and said the drug will be made available to patients under conditions that include brain imaging and ongoing safety monitoring.
Donanemab, which targets amyloid-beta plaques in the brain, demonstrated in the pivotal TRAILBLAZER-ALZ 2 trial a slowing of clinical decline among participants with early Alzheimer’s who had biomarker evidence of amyloid. The trial also showed substantial reductions in amyloid levels on positron emission tomography scans, findings that helped persuade regulators to approve the drug for patients with mild cognitive impairment or mild dementia attributed to Alzheimer’s disease.
“Today’s approval offers a new option for people in the earliest stages of Alzheimer’s,” an Eli Lilly spokesperson said in a statement. The company intends to work with health systems and payers to implement the necessary diagnostic and monitoring protocols.
FDA approval comes with firm caveats. Regulators emphasized the need for careful screening and serial neuroimaging because donanemab is associated with amyloid-related imaging abnormalities, or ARIA, a spectrum of side effects that can include brain swelling and microhemorrhages. To manage those risks, the approval requires pre-treatment magnetic resonance imaging and periodic follow-up MRIs — a practice underscored by clinicians who rely on MRI films to spot subtle changes. Physicians have already begun retooling clinic workflows to incorporate these scans into patient selection and follow-up.
Outside experts welcomed the decision cautiously, noting that while the drug’s effect size represents a meaningful shift for some patients, it is not a cure. “This is a step forward, but we must temper expectations,” said an independent neurologist familiar with the data. “The benefit in clinical function, while real, is modest and must be balanced against the risk of ARIA and the practical burdens of diagnosis and monitoring.”
The approval will intensify longstanding debates about cost and access. The price for donanemab has not yet been announced, but prior experience with amyloid-targeting therapies prompted contentious negotiations over Medicare coverage and out-of-pocket costs. Widespread use will also drive demand for amyloid testing — via PET scans or cerebrospinal fluid assays — and MRI capacity, potentially straining resources in community clinics and rural hospitals.
For patients and caregivers, the decision brings mixed emotions: relief at a new therapeutic option, and uncertainty about who will benefit most. Alzheimer’s advocacy groups praised the availability of another tool for early-stage disease while urging insurers and policymakers to ensure equitable access.
Regulators required additional postmarketing studies to better define long-term clinical outcomes and safety in broader populations. Clinicians say those results and real-world experience will be crucial to refining treatment protocols, including identifying which patients have the best risk-benefit profile.
As neurologists pore over MRI films and biomarker panels, the approval signals a new era in Alzheimer’s care — one in which precision diagnostics and monitored immunotherapy may become standard, even as questions remain about affordability, equity and the ultimate impact on the course of the disease.