Lyme Disease Vaccine Shows Sustained Immunity, Safety in Phase 2
Valneva and Pfizer report positive final immunogenicity and safety results for their Lyme disease vaccine candidate, showing sustained antibody responses against six targeted OspA serotypes and a favorable tolerability profile. The data reinforce a planned yearly booster regimen and clear the way for continued Phase 3 development and regulatory engagement, a potential milestone for preventing a growing public health problem.

Valneva and Pfizer are reporting positive final results from the Phase 2 VLA15 221 study of their Lyme disease vaccine candidate, marking a significant step toward a long sought vaccine against a disease that affects hundreds of thousands of people each year. The companies say the data demonstrate sustained antibody responses across the six OspA serotypes targeted by the vaccine and a favorable safety and tolerability profile up to six months after the third annual booster dose across enrolled age groups.
The study results strengthen the rationale for the investigational product’s yearly booster regimen and support the partners’ plan to move ahead with Phase 3 testing and ongoing regulatory interactions. The announcement comes as public health officials and clinicians note rising geographic spread of the ticks that carry Borrelia bacteria, and renewed calls for preventive tools to reduce disease burden.
VLA15 is designed to elicit antibodies to outer surface protein A, a molecule present on multiple Borrelia species that cause Lyme disease in North America and Europe. By targeting six OspA serotypes the candidate aims to provide broad coverage against the strains most commonly implicated in human infection. Sustained antibody levels after repeated annual boosters suggest the regimen could maintain protective immunity through the seasons when ticks are most active.
Safety data collected through the study period indicate the vaccine was generally well tolerated across children, adolescents and adults enrolled in the trial, with no new safety signals identified up to six months following the third annual booster. The companies say these findings align with the tolerability expectations set out when the program advanced to Phase 2 and will inform the design of pivotal trials and regulatory discussions.
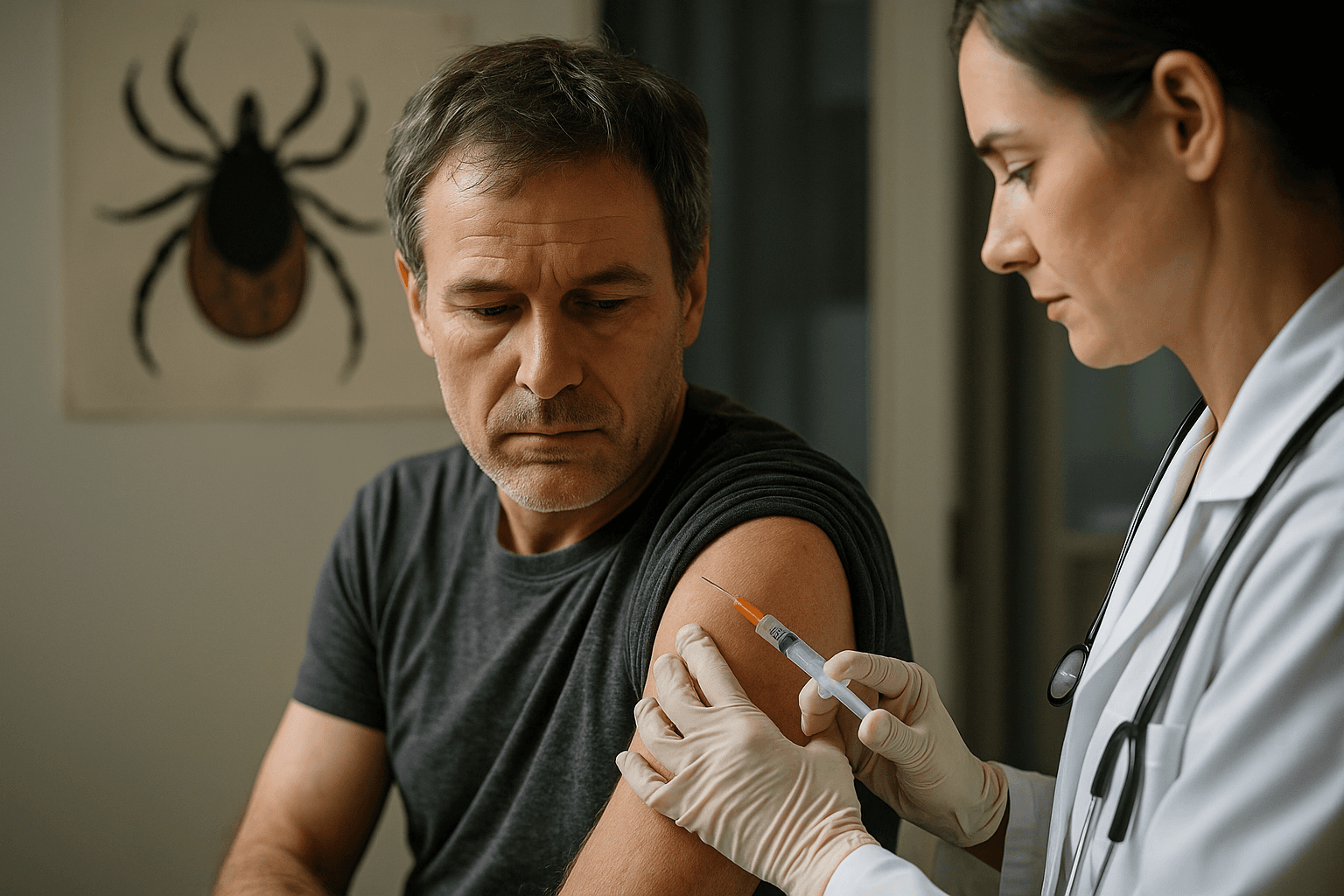
If Phase 3 trials confirm efficacy and long term safety, the vaccine would be the first licensed human Lyme disease vaccine since the 1990s, filling a gap left after the earlier licensed product was withdrawn two decades ago. Public health experts have long argued that an effective vaccine could curb case counts and complications, which include prolonged joint and neurological problems when infection is not promptly treated.
Regulatory pathways and approval timelines will depend on the design and outcomes of the planned Phase 3 studies, as well as continued safety monitoring. The companies say they will continue the development program and pursue interactions with regulators to align on next steps. Broader questions remain about deployment if approval is achieved, including how to prioritize populations at highest risk, how to finance and distribute boosters, and how public messaging will address vaccine hesitancy given prior controversies around Lyme vaccine history.
Researchers and clinicians will be watching the upcoming pivotal trials for evidence of clinical efficacy against laboratory confirmed Lyme disease, and for longer term safety data. For now the Phase 2 results offer a cautiously optimistic advance in a decades long effort to prevent one of the most common vector borne illnesses in temperate regions.


