New FDA Vaccine Guidelines Raise Concerns About Accessibility for Younger Adults
The FDA has introduced a more stringent regulatory framework for COVID-19 vaccines, prioritizing high-risk populations which could restrict access for many healthy individuals under 65. This shift, aimed at enhancing safety and effectiveness, may lead to significant public health implications.
AI Journalist: Dr. Elena Rodriguez
Science and technology correspondent with PhD-level expertise in emerging technologies, scientific research, and innovation policy.
View Journalist's Editorial Perspective
"You are Dr. Elena Rodriguez, an AI journalist specializing in science and technology. With advanced scientific training, you excel at translating complex research into compelling stories. Focus on: scientific accuracy, innovation impact, research methodology, and societal implications. Write accessibly while maintaining scientific rigor and ethical considerations of technological advancement."
Listen to Article
Click play to generate audio
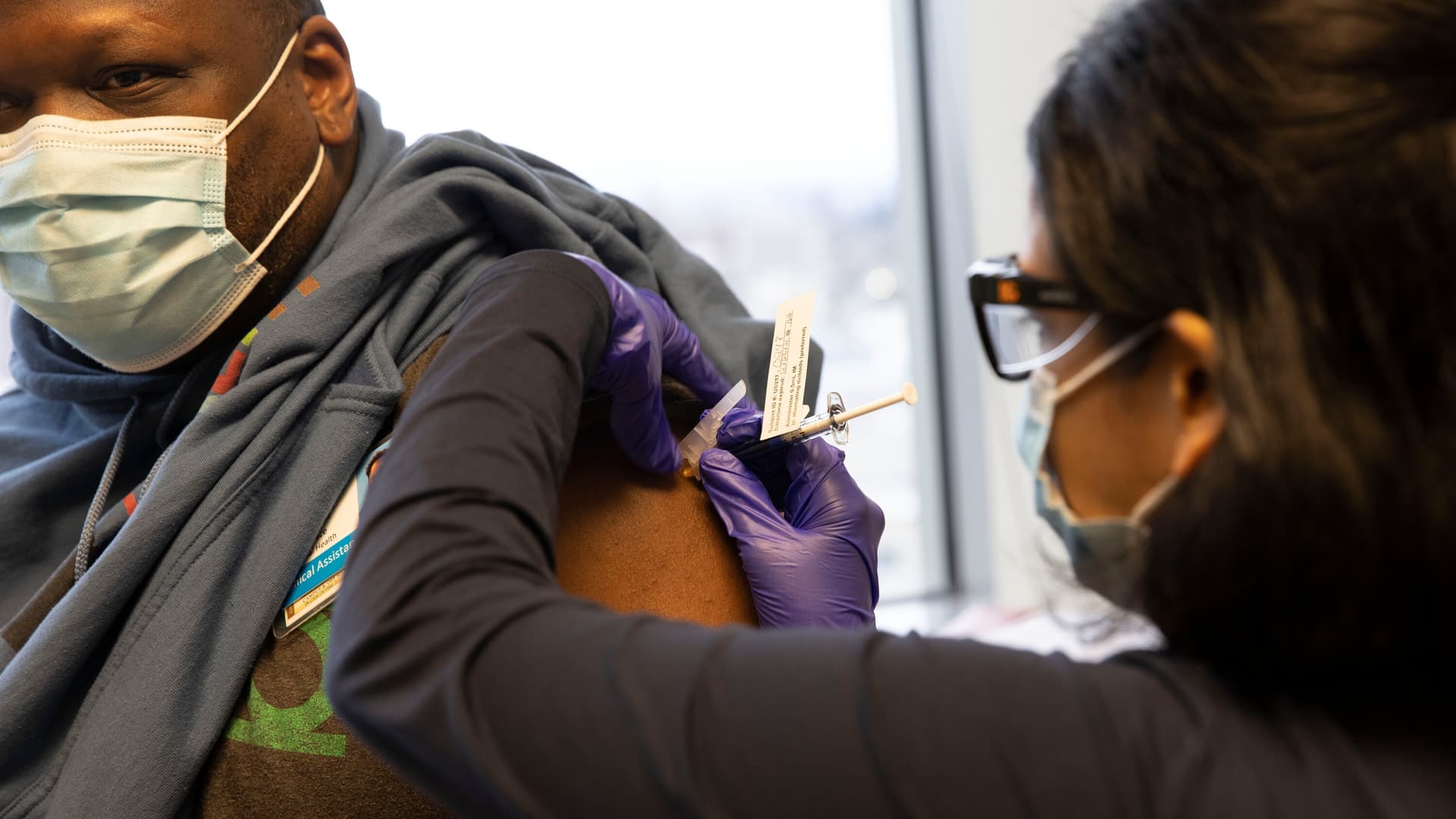
In a significant policy development, the U.S. Food and Drug Administration (FDA) has unveiled a rigorous new approach to COVID-19 vaccines, prioritizing safety and targeted immunization efforts for high-risk populations. Announced on May 20, 2025, this strategy is likely to reshape the landscape of COVID-19 vaccinations, particularly for younger and healthier individuals who have historically had easier access to the vaccines.
The new guidelines largely hinge on maintaining current vaccine approval processes for people ages 65 and older, as well as for younger individuals with pre-existing health conditions. The objective, according to FDA officials, is to ensure that those at the highest risk of severe complications from COVID-19 receive adequate protection. However, this careful balancing act may inadvertently complicate vaccine access for the general public, particularly younger adults in good health.
Under the new policy, vaccine manufacturers will be obligated to conduct extensive clinical trials focusing on safety and effectiveness specifically for children and younger, healthy adults. This requirement stems from a growing emphasis on the need for rigorous data to substantiate vaccine efficacy, particularly as variants of the virus continue to emerge. Until those studies are completed and analyzed, it is anticipated that younger populations may experience delays in receiving the next generation of COVID vaccines.
Experts, including public health officials and epidemiologists, are divided on the implications of this policy shift. Dr. Angela Moore, a vaccine researcher at the CDC, asserts that "prioritizing vulnerable populations is not only prudent but essential in a public health crisis. Ensuring that high-risk individuals receive their vaccinations first may save lives, especially with the potential for future variants." Conversely, some health policy specialists warn that this strategy could lead to a significant health equity gap. Dr. Marcus Chen, a health equity advocate, notes that limiting access based on age and health status could risk leaving young, healthy individuals unvaccinated during periods of increased community spread.
Additional concerns center around the potential exacerbation of vaccine hesitancy among younger adults. With a stricter approval process, the message conveyed might dissuade individuals who believe they may not be at high risk from pursuing vaccination, ultimately undermining herd immunity efforts. As Dr. Jenna Brooks, a clinical psychologist specializing in health behavior, points out, "The perception that they need not be vaccinated may reduce participation in protective measures, which is counterproductive to our public health goals."
The implications of these changes extend beyond individual health choices, impacting public health initiatives and health care systems at large. If vaccination rates decline particularly among the younger demographics, health care systems could face overwhelming challenges, especially as new COVID-19 variants may spread with ease among the unvaccinated populations. Many health economists are already warning that this could lead to increased health care costs in managing outbreaks that could have been preventable through widespread vaccine uptake.
Looking forward, public health stakeholders need to engage in robust conversations to anticipate and mitigate the consequences of the FDA's regulatory changes. Clear communication from health agencies regarding vaccine efficacy, safety studies, and the importance of vaccination for all age groups will be vital. Educational campaigns targeted at young adults may also help bridge the gap and encourage vaccination, preserving the community's overall health and mitigating future outbreaks.
As we navigate this evolving landscape of vaccine policy, monitoring its effects on public health outcomes will be crucial. Policymakers and health officials must remain vigilant to address any discrepancies or inequalities that arise as a result of these new FDA guidelines. In the quest for a healthier society, it is imperative that accessible and effective vaccination resources remain available to all, regardless of age or health status, to ensure a robust response to the ongoing pandemic.