Botulism outbreak linked to ByHeart formula reaches 51 infants
Federal health agencies expanded an investigation tying infant botulism to ByHeart Whole Nutrition formula, now counting at least 51 cases in 19 states. The widening probe raises new questions about product safety, testing practices, and access to limited antitoxin treatment for affected families.

Federal officials said Wednesday that the multistate outbreak of infant botulism connected to ByHeart Whole Nutrition infant formula now includes at least 51 infants across 19 states, after the Centers for Disease Control and Prevention broadened its case definition to capture any infant exposed to the product since it was first produced. The CDC and the Food and Drug Administration said no deaths have been reported, and the most recent illness was reported on December 1, 2025.
ByHeart began producing the Whole Nutrition formula in March 2022. Health authorities first announced a cluster of botulism cases linked to the powdered product on November 8, 2025, prompting an initial voluntary recall by the company. Following requests from federal and state officials, ByHeart expanded that recall on November 11, 2025 to include all cans and single serve Anywhere Pack sticks sold nationwide. The FDA has since requested a formal nationwide recall expansion.
The updated count released December 10 reflects a change in how the CDC measures the outbreak. The agency broadened its case definition to include “any infant with botulism who was exposed to ByHeart formula at any time since the product’s release,” adding ten illnesses that occurred between December 2023 and July 2025 as well as two additional cases within the original monitoring period. Earlier federal updates had reported 39 suspected or confirmed cases across 18 states, with cases counted from August 1, 2025 onward.
Laboratory testing by federal, state and independent labs has detected Clostridium botulinum, the bacterium that can produce botulinum toxin, in multiple samples associated with ill infants. California public health officials confirmed that an open can of ByHeart formula linked to an ill infant contained the bacterium. California officials described the standard confirmatory test, which involves injecting cultured bacteria into mice and observing for illness over up to four days, and said the mice “got sick really quickly.”
ByHeart disclosed on November 19 that it had tested 36 finished product samples across three lots and that five samples tested positive for C. botulinum Type A. Earlier company statements had said no unopened product had tested positive, a discrepancy that federal investigators and independent laboratories are continuing to examine as part of a broader FDA and CDC investigation into a larger spike in infant botulism this year.
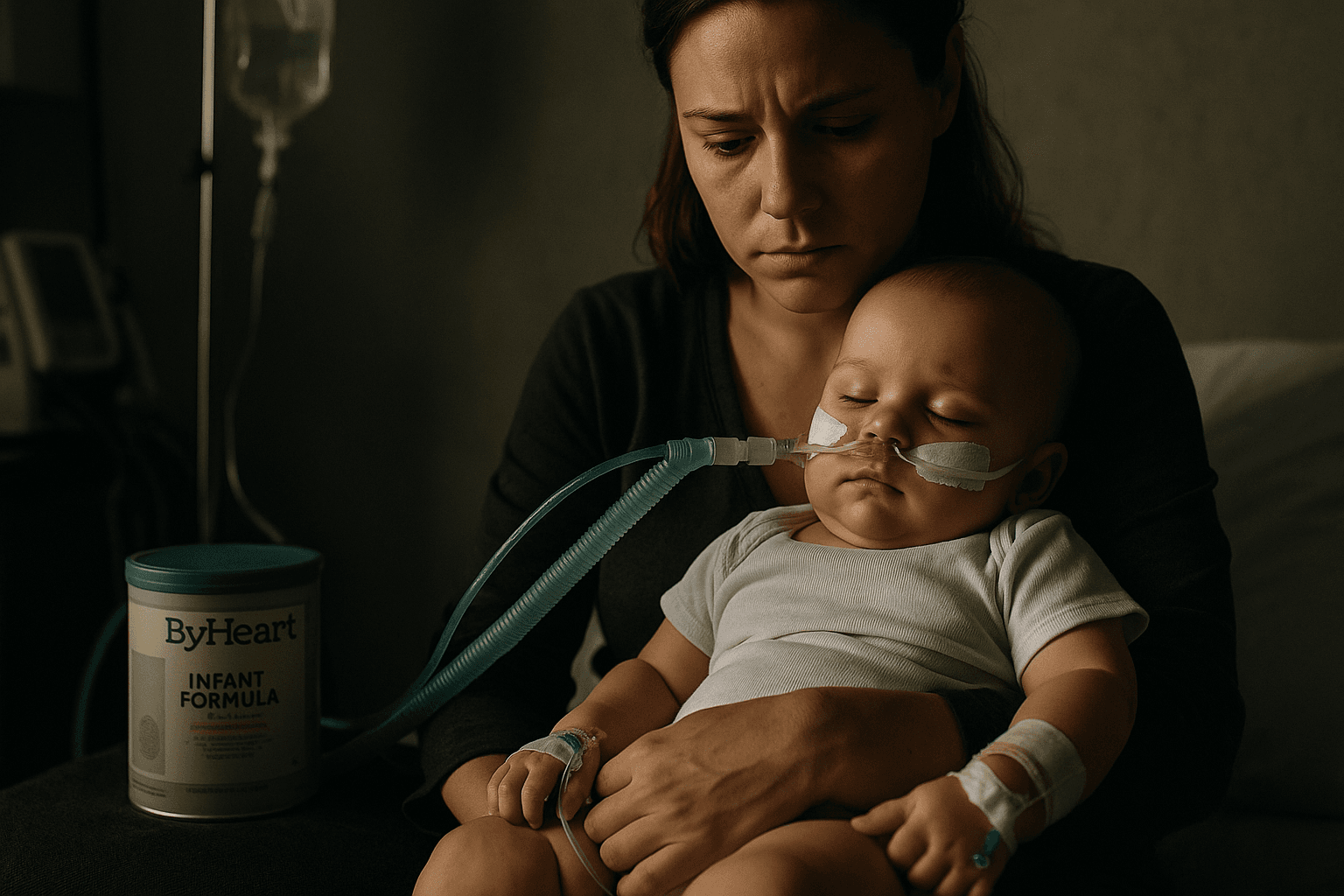
FDA inspections of ByHeart manufacturing sites in Iowa and Oregon remain under way, and the agency is conducting additional sampling and a review of manufacturing practices. The FDA has urged consumers to stop using and to dispose of any ByHeart Whole Nutrition cans and Anywhere Pack products.
Clinicians treating affected infants have relied on BabyBIG, a human immune globulin antitoxin distributed through California’s Infant Botulism Treatment and Prevention Program, but supply is limited. Hospitals and public health partners across multiple states have coordinated to secure treatment for infants. Families of some affected infants have filed lawsuits alleging the ByHeart formula caused the illnesses.
Investigators said testing, traceback and facility inspections are ongoing, and federal officials warned that further updates about implicated lots, root causes and regulatory actions were likely as the probe continues.
Know something we missed? Have a correction or additional information?
Submit a Tip