FDA Warns Major Retailers After Recalled ByHeart Infant Formula Sold
The Food and Drug Administration issued warning letters to Walmart, Target, Kroger and Albertsons after inspectors found recalled ByHeart Whole Nutrition infant formula remained available for purchase at multiple stores for days or weeks following a mid November recall. The letters underscore public health risks from delayed removals and raise tough questions about retailer recall procedures and protections for families who depend on infant formula.

The U.S. Food and Drug Administration has formally warned four of the nation’s largest retail chains after finding that ByHeart Whole Nutrition infant formula subject to a botulism related recall remained on store shelves long after the recall was announced in mid November. The warning letters, dated December 12 and issued by the FDA’s Human Foods Program, were posted publicly in mid December and require the companies to document corrective actions within 15 working days.
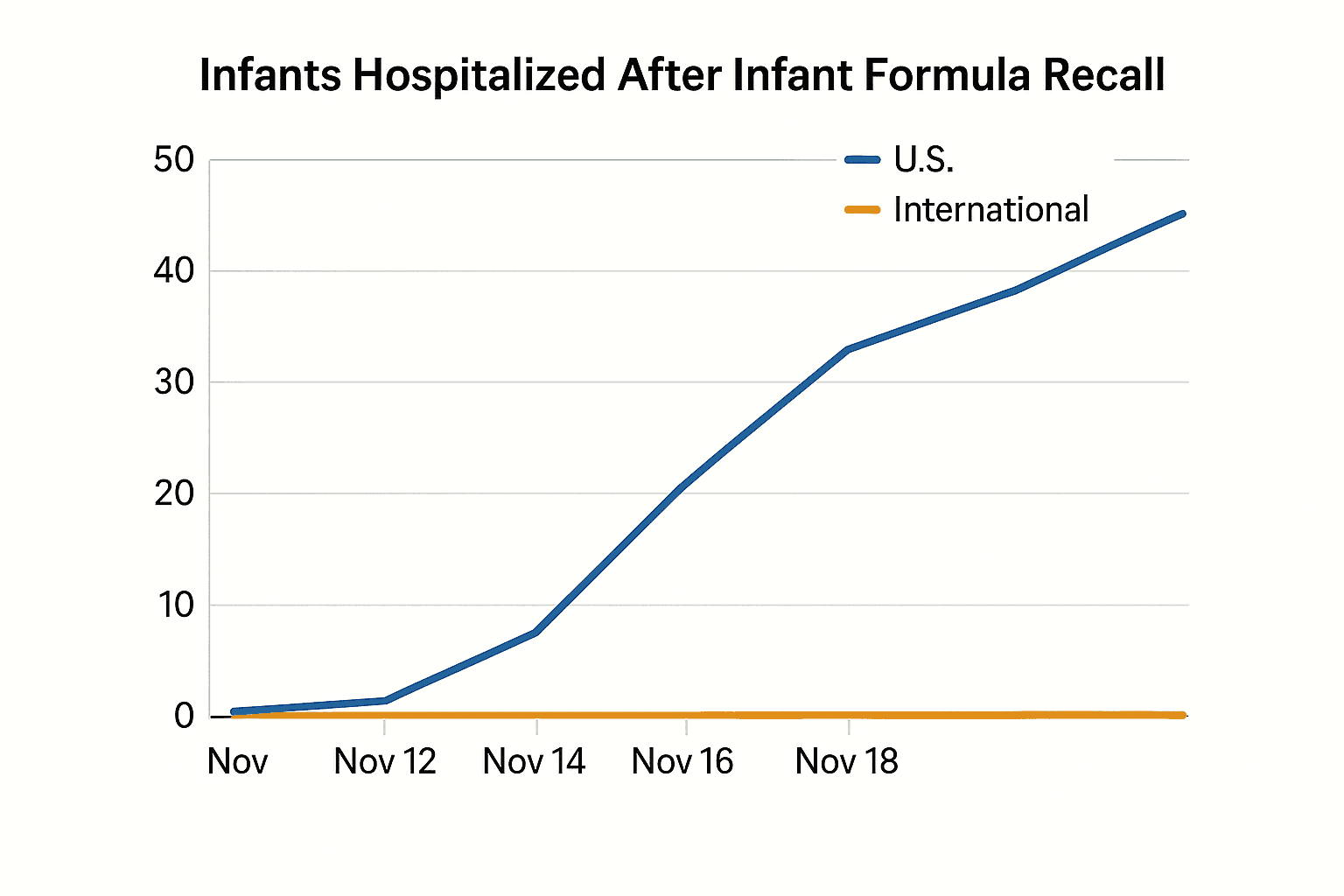
The recall followed a cluster of infant botulism cases that health authorities say led to more than 50 infants being hospitalized across 19 states. Separately, international tallies as of November 20 indicated dozens of infants had been affected in multiple countries. Public health officials have stressed the severity of infant botulism and the urgent need to remove potentially contaminated formula from commerce to prevent further harm.
The FDA’s correspondence to the retailers cites evidence that recalled product remained available for sale at numerous locations for an extended period after the recall notice. The FDA reminded the firms that “the receipt in interstate commerce of adulterated food, and the delivery or proffered delivery thereof for pay or otherwise, is a violation” of federal law and emphasized that supply chain participants must “take prompt and effective action when notified of a product recall.” An industry wide letter accompanying the individual notices reiterated that businesses are required by law to maintain written recall plans that assign responsibility and lay out steps to be taken.

Kroger was cited based on state and local partner information showing recalled ByHeart formula remained on shelves at Kroger locations across 10 states from November 12 to November 19, though specific store addresses were redacted in the public release. The FDA released the full warning letter to Walmart and posted partly redacted letters for Target and Albertsons. In response to agency inquiries, an Albertsons spokesperson said, “ByHeart infant formula products have been removed from our store shelves.”
The agency has asked each retailer to provide documentation demonstrating the scope of the problem at their stores, actions taken to remove the product, and plans to prevent recurrence. The 15 working day response deadline triggers potential enforcement steps if the FDA finds the firms’ corrective measures inadequate.
Public reaction has been immediate and anguished. Families and health advocates have called for greater transparency and faster corrective action when infant foods are implicated in a safety event. Advocates note that delays in removing contaminated formula can disproportionately harm low income families and those with limited access to pediatric care, compounding the public health toll.
The episode has also highlighted strain points in formula supply chains and recall protocols. Experts in food safety say the incident will likely prompt closer scrutiny of retailer recall practices, including how stores are notified, how inventory is flagged and how staff are trained to execute written recall plans. For parents and caregivers, the FDA and pediatricians continue to advise checking recall notices and contacting local health authorities or pediatric providers if there are any concerns about infant feeding or symptoms consistent with botulism.
Know something we missed? Have a correction or additional information?
Submit a Tip