FDA Begins Fresh Safety Review of Infant RSV Antibody Therapies
U.S. regulators notified Merck, Sanofi and AstraZeneca on December 9 that the Food and Drug Administration will conduct new safety reviews of long acting antibody therapies used to prevent respiratory syncytial virus in infants. The move, described by the FDA as routine, matters because these drugs have been credited with reducing hospitalizations even as immunization policy faces close scrutiny under Health Secretary Robert F. Kennedy Jr.

The Food and Drug Administration on December 9 informed senior executives at Merck, Sanofi and AstraZeneca that it would open fresh safety reviews of their approved long acting antibody therapies for infants, agency officials said. The reviews will examine the safety profiles of Merck’s Enflonsia and Sanofi and AstraZeneca’s Beyfortus, products that have been widely adopted to prevent severe respiratory syncytial virus infection in young children.
The agency characterized the process as a routine reexamination and said it would update product labeling if new information warranted that step. Company statements provided to regulators said they had not identified any new safety signals. The communication follows concerns raised by some outside advisers and commentators and comes amid a broader reassessment of immunization policy led by Health Secretary Robert F. Kennedy Jr.
The therapies at the center of the review are long acting antibody treatments, administered to infants to provide passive protection against RSV during peak seasons. Clinical and real world data have been credited with reducing RSV related hospitalizations among infants, a key public health achievement after a particularly severe respiratory season put pressure on pediatric services. The FDA review does not indicate that new harms have been found, but it reflects heightened vigilance over vaccines and antibody products in the current political and regulatory environment.
Public health officials and pediatric clinicians said the review could prompt closer guidance for clinicians and may affect how hospitals and pediatric practices counsel families during the coming season. Manufacturers and some clinicians have argued that any pause or heightened scrutiny must be balanced against the clear benefits observed in preventing severe illness and limiting intensive care admissions.
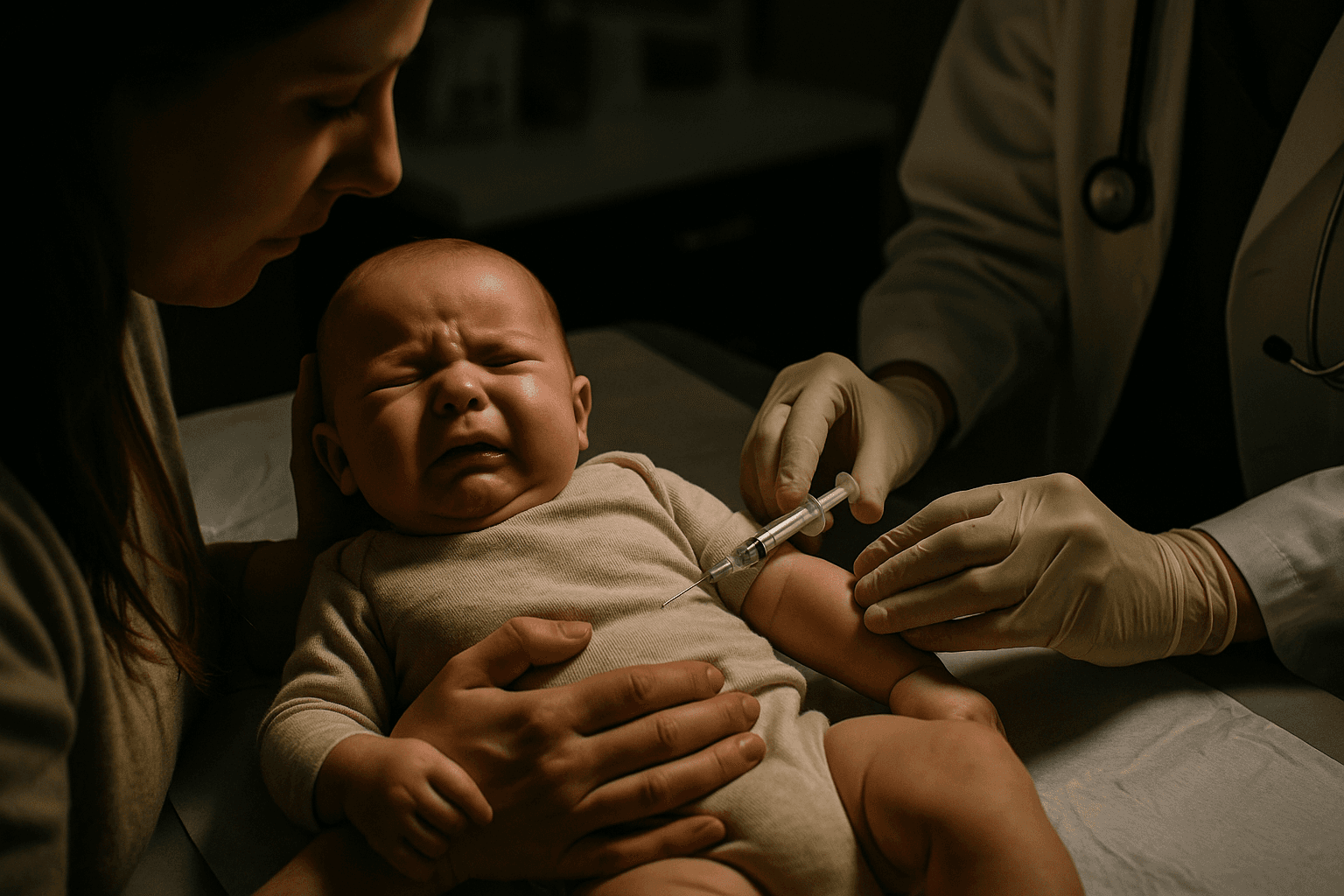
The timing of the inquiry adds a layer of uncertainty because it coincides with broader policy shifts at the Department of Health and Human Services. Since taking office, the health secretary has initiated reviews of vaccine and immunization policies, raising questions among industry and public health communities about how scientific findings will be weighed against political and public perceptions. The FDA is an independent regulator within that landscape, and agency officials emphasized the routine nature of the review while promising transparency if new safety concerns emerge.
Parents and caregivers will be watching whether the reviews lead to new product labeling or changes in clinical recommendations. For now, companies maintain there are no new safety signals and public health authorities continue to recommend prevention strategies for infants at highest risk of severe RSV.
The renewed scrutiny highlights the delicate balance regulators must strike between maintaining public confidence in preventive therapies and ensuring that emerging safety information is investigated thoroughly. Reporting by Dan Levine and Patrick Wingrove.
Sources:
Know something we missed? Have a correction or additional information?
Submit a Tip

