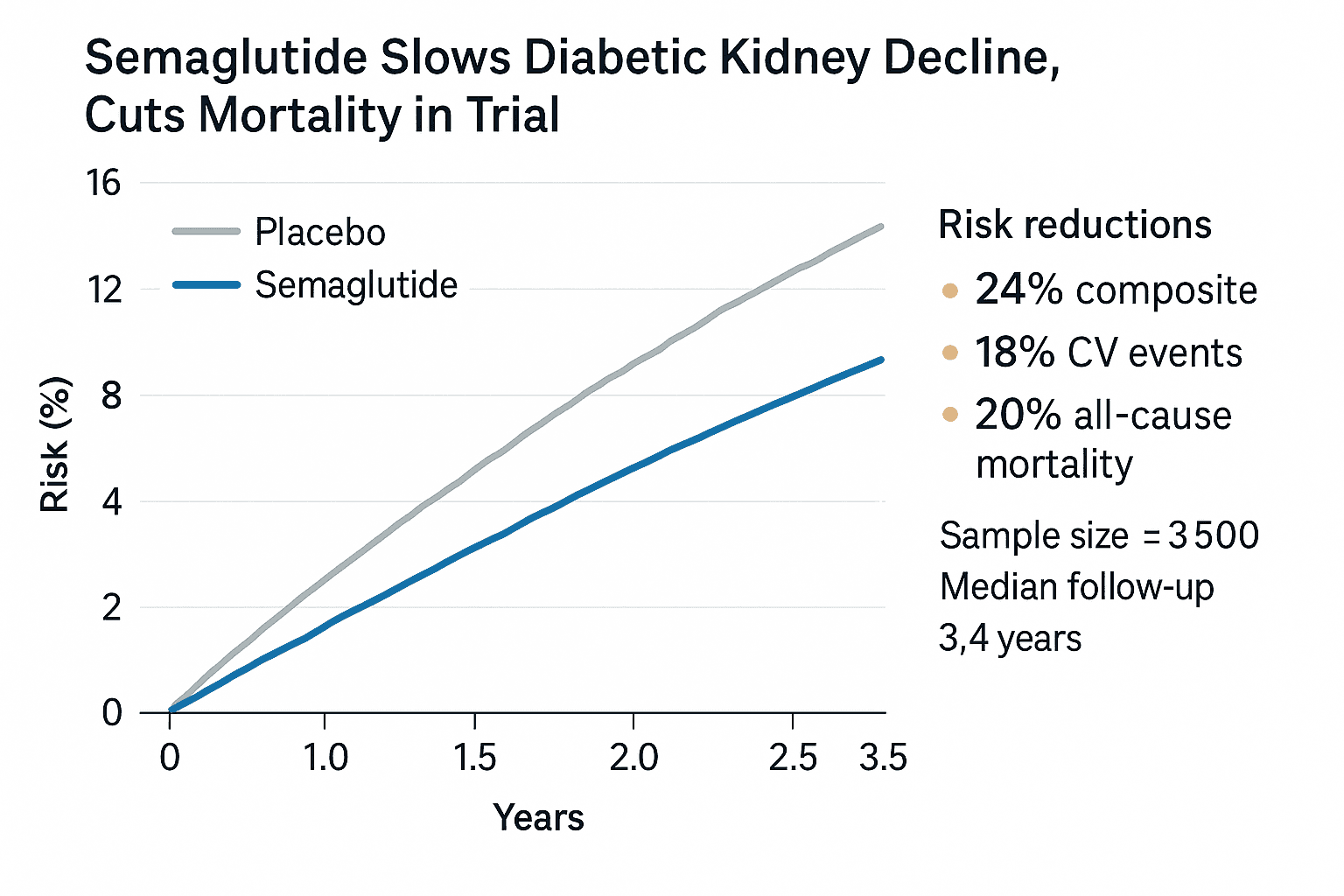
Semaglutide Slows Diabetic Kidney Decline, Cuts Mortality in Trial
A large international randomized trial found once weekly semaglutide reduced major kidney events and death in people with type 2 diabetes and chronic kidney disease, suggesting the drug can meaningfully slow kidney decline. The result, published December 5 in the New England Journal of Medicine and presented at an international nephrology meeting, could prompt rapid changes in clinical guidelines, insurance coverage, and drug labeling.

A multinational clinical trial known as FLOW showed that once weekly injectable semaglutide at a 1.0 milligram dose reduced the risk of a composite of major kidney events and kidney or cardiovascular related death by 24 percent compared with placebo in patients with type 2 diabetes and chronic kidney disease. The randomized, multicenter study enrolled roughly 3,500 participants across about 28 countries and followed them for a median of about 3.4 years before an independent data monitoring committee recommended stopping early for clear benefit.
Beyond the primary composite outcome, the trial reported slower decline in estimated glomerular filtration rate, an approximately 18 percent reduction in major adverse cardiovascular events, and an approximately 20 percent reduction in all cause mortality among those assigned to semaglutide. Investigators presented the results at an international nephrology conference and published detailed findings in the New England Journal of Medicine on December 5.
The magnitude and breadth of the effects mark a notable development in the treatment of diabetic kidney disease, a leading cause of kidney failure and a major contributor to cardiovascular mortality in people with diabetes. Current standard care for people with diabetes and kidney disease includes blood pressure control, blockade of the renin angiotensin system, and sodium glucose cotransporter 2 inhibitors for eligible patients. FLOW provides evidence that a glucagon like peptide 1 receptor agonist can add kidney protective effects over several years of treatment.

Semaglutide is already widely prescribed for glycemic control and weight management under brand names including Ozempic and Wegovy. The FLOW results are likely to accelerate discussions among guideline panels about whether semaglutide should be recommended specifically for kidney protection in patients with type 2 diabetes and chronic kidney disease. Regulatory agencies and payers will also be watching, because the trial outcomes could affect labeling, coverage determinations, and prescribing patterns across multiple indications and products.
Clinicians and researchers caution that questions remain about how semaglutide produces kidney benefit. It is not yet clear to what extent effects derive from improved blood glucose control, weight loss, reductions in blood pressure or inflammation, or direct actions on kidney tissue. The trial data include measures of kidney function decline but do not by themselves establish mechanism, and further analyses and mechanistic studies will be needed.
Safety and tolerability data from the trial will be closely examined by regulators and clinicians to weigh benefits against known adverse effects associated with GLP 1 receptor agonists. Given the early stopping for benefit and the magnitude of cardiovascular and mortality reductions reported, the findings are expected to prompt rapid reassessment of treatment strategies for millions of people living with type 2 diabetes and chronic kidney disease.
As medical societies consider revisions and payers review coverage policies, patients and clinicians may soon have a new option for reducing the progression of diabetic kidney disease and its lethal complications.