CRISPR Therapy Casgevy Shows Promise for Young Children with Blood Disorders
Vertex Pharmaceuticals reported early clinical results showing its CRISPR based gene therapy Casgevy eliminated painful sickle cell events in children aged 5 to 11 and freed children with transfusion dependent beta thalassemia from blood transfusions for at least 12 consecutive months. The findings, the first clinical data for any gene therapy in this younger sickle cell population, could expand access to a potentially curative option and reshape care for children with severe inherited blood disorders.
Vertex Pharmaceuticals announced on December 6 that early clinical data for its gene therapy Casgevy showed striking benefits in children aged 5 to 11 with sickle cell disease and transfusion dependent beta thalassemia. According to the company, children with sickle cell disease in the study became free of painful vaso occlusive events, while children with transfusion dependent beta thalassemia were maintained without blood transfusions for at least 12 consecutive months. The results represent the first clinical evidence for any gene therapy in children this young with sickle cell disease and support potential expansion of Casgevy into a younger age group.
Casgevy is developed using CRISPR gene editing technology and is already approved for patients aged 12 and older with sickle cell disease or transfusion dependent beta thalassemia. The new data, described by Vertex as early, point to the possibility that treating patients earlier in life could prevent the accumulation of organ damage and other complications that arise from repeated pain crises and chronic transfusion therapy. For families, a durable, one time treatment could mean fewer hospitalizations, less missed school, and relief from the lifelong burden of intensive disease management.
Medical experts caution that early data do not yet establish long term safety and durability. Gene editing therapies involve complex manufacturing, and responses established over a year in a small cohort will need confirmation in larger studies and longer follow up. Regulators will review safety and efficacy data before any label expansion for younger children. The announcement is nevertheless notable for its timing and scope, because sickle cell disease disproportionately affects children in marginalized communities and current treatments for severe disease can be onerous and limited.
Beyond clinical outcomes, the Casgevy data raise broader questions about access and equity. Gene therapies are costly to develop and deliver. Intravenous cell processing and bespoke manufacturing add barriers to rapid adoption in routine pediatric practice. Health systems, insurers, and policymakers will face difficult decisions about who receives early access and how to finance potentially curative but expensive interventions. Advocates and clinicians have argued for payment models that spread cost over time and link reimbursement to long term outcomes.
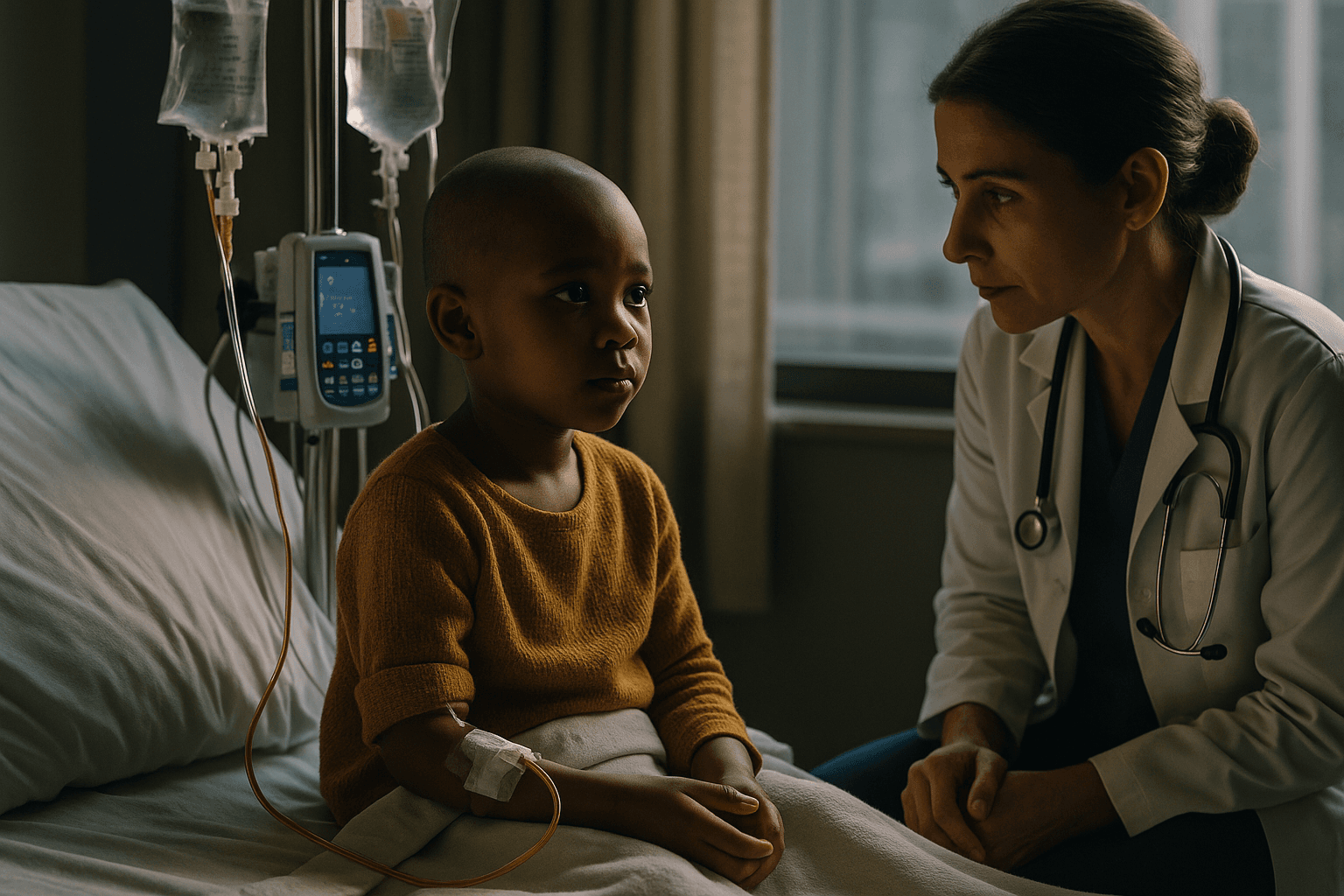
Ethical considerations also accompany expansion of gene editing into younger populations. While Casgevy modifies patients own blood stem cells rather than altering germline DNA, treating children introduces additional consent and risk benefit complexities. Long term monitoring for off target effects and unexpected late complications will be essential.
If subsequent larger and longer studies confirm these early results, Casgevy could mark a turning point for treatment of severe inherited blood disorders in children. For now, the data announced by Vertex offer hope and also underscore the substantial clinical, logistical, and ethical work that lies ahead before CRISPR based therapies become routine care for young patients.


