Vertex gene therapy reduces painful events in young children, shows promise
Vertex Pharmaceuticals announced positive trial results for a gene therapy in children aged five to eleven with sickle cell disease and another transfusion dependent blood disorder, reporting fewer painful events and some patients becoming transfusion free for at least 12 consecutive months. The findings raise hopes for changing pediatric care, while also highlighting the need for long term safety monitoring, equitable access, and policy planning to ensure communities most affected see benefits.

Vertex Pharmaceuticals said on December 6, 2025 that a gene therapy examined in clinical trials produced meaningful clinical benefits in children aged five to eleven with sickle cell disease and another transfusion dependent blood disorder. According to the company, children treated in the study experienced fewer painful vaso occlusive events or, in some cases, became transfusion free for at least 12 consecutive months. The announcement marks one of the earliest demonstrations of gene therapy benefit in this younger pediatric population for these chronic disorders.
For families and clinicians, the results suggest a possible shift in how severe childhood blood disorders are treated. Sickle cell disease is a lifelong condition that often causes recurrent pain crises, hospitalizations, and cumulative organ damage. Transfusion dependent disorders require repeated blood transfusions with attendant risks and burdens, including iron overload and frequent clinic visits. Reducing painful episodes or eliminating the need for transfusions for an extended period could improve quality of life, school attendance, and long term health trajectories for affected children.
Public health experts caution, however, that the promise of early clinical success must be weighed against outstanding questions. Long term safety and durability of benefit will be central to understanding whether gene therapy represents a durable cure or a temporizing treatment. Regulators will want extended follow up to monitor for late adverse events and to confirm that benefits persist as children grow. Manufacturing scale up and consistent product quality are additional practical hurdles before broad clinical adoption.
The announcement also foregrounds long standing equity and access concerns. Sickle cell disease disproportionately affects Black and Latino communities in the United States and is common in other regions globally. Historically these communities have experienced gaps in access to specialized care and have often been underserved by high cost innovations. If gene therapies become available for pediatric patients, policymakers and payers will face hard choices about coverage, pricing, and distribution to prevent widening disparities. Expanded Medicaid coverage, risk sharing agreements, and federal programs could be needed to ensure that breakthroughs do not remain accessible only to those with the deepest resources.
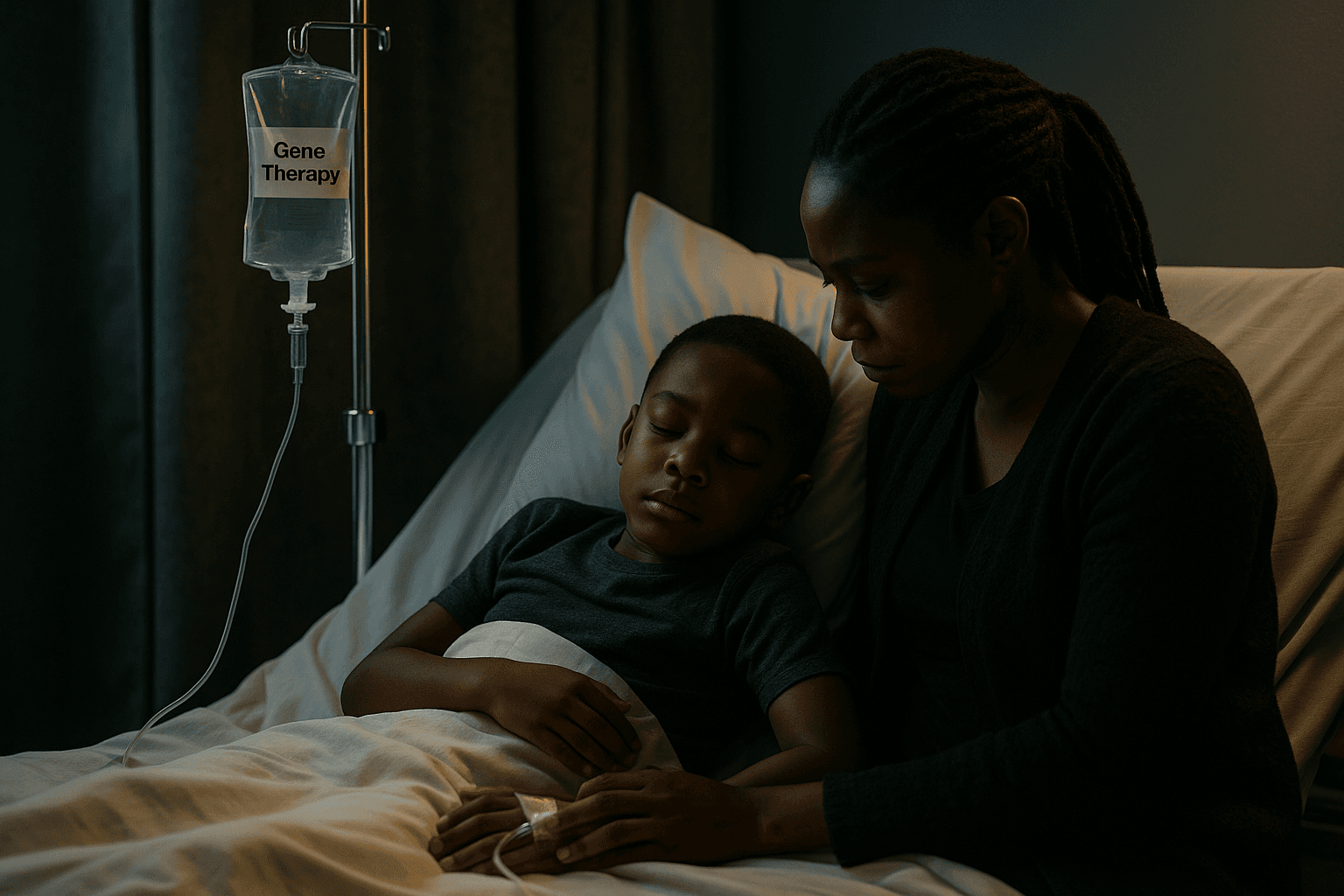
Community engagement and trust building will be crucial as developers pursue regulatory filings and additional studies. Families balancing the risks of a novel cellular therapy against the chronic harms of disease will need clear information, culturally competent counseling, and support to weigh options. Public health systems should prepare for changes in demand for transfusion services and pediatric hematology care, while also investing in surveillance systems that capture long term outcomes across diverse populations.
Vertex said it will pursue next regulatory and development steps following the trial outcomes. As the company advances its program, the broader health system will need to align scientific enthusiasm with safeguards for safety, affordability, and equity so that the potential benefits reach the children and communities who need them most.