FDA, Abbott Alert Patients About FreeStyle Libre 3 False Low Readings
The U.S. Food and Drug Administration and Abbott issued a safety communication after certain lots of the FreeStyle Libre 3 continuous glucose monitoring sensors were found to produce falsely low glucose readings, a problem linked to seven deaths and hundreds of injuries overall. The warning matters because many people with diabetes rely on continuous monitors to dose insulin and make daily decisions, leaving some particularly vulnerable to potentially life threatening undertreatment.
The U.S. Food and Drug Administration and Abbott on December 7 issued a safety communication warning that specific lots of the FreeStyle Libre 3 continuous glucose monitoring sensors can produce falsely low glucose readings. Abbott and the FDA said the problem has been associated with multiple serious adverse events, with reported deaths occurring outside the United States and an aggregate tally of seven deaths and hundreds of injuries tied to the issue.
Abbott urged users to stop using affected lots and to verify their sensor lot numbers against instructions the company provided, and it offered replacement sensors and customer support for patients who depend on its systems. The FDA said it is working with Abbott to ensure appropriate corrective actions and broader communication to patients and health care providers.
Continuous glucose monitors have transformed diabetes care by reducing the need for frequent fingerstick testing and by making real time glucose trends accessible. For many insulin dependent people, however, the devices are not merely convenient, they are central to daily safety. A sensor that reports a falsely low value can prompt a patient or caregiver to overtreat presumed hypoglycemia, or conversely can obscure high glucose levels if users doubt the device and delay treatment. Either scenario can lead to severe complications.

Clinicians were advised by Abbott and the FDA to confirm glucose values with fingerstick testing when a patient’s symptoms do not match the sensor reading and to contact Abbott for replacement sensors from unaffected lots. The agencies emphasized continued vigilance in reporting adverse events to the FDA so that regulators can track the scope of the problem and monitor corrective measures.
The episode spotlights broader public health and equity concerns. Low income patients and communities of color are less likely to have consistent access to backup testing supplies such as fingerstick meters and test strips, and they are more likely to face financial and logistical barriers when a critical device must be replaced. Insurance coverage gaps for replacement sensors and essential supplies can compound risk, leaving already vulnerable patients with fewer safe options.
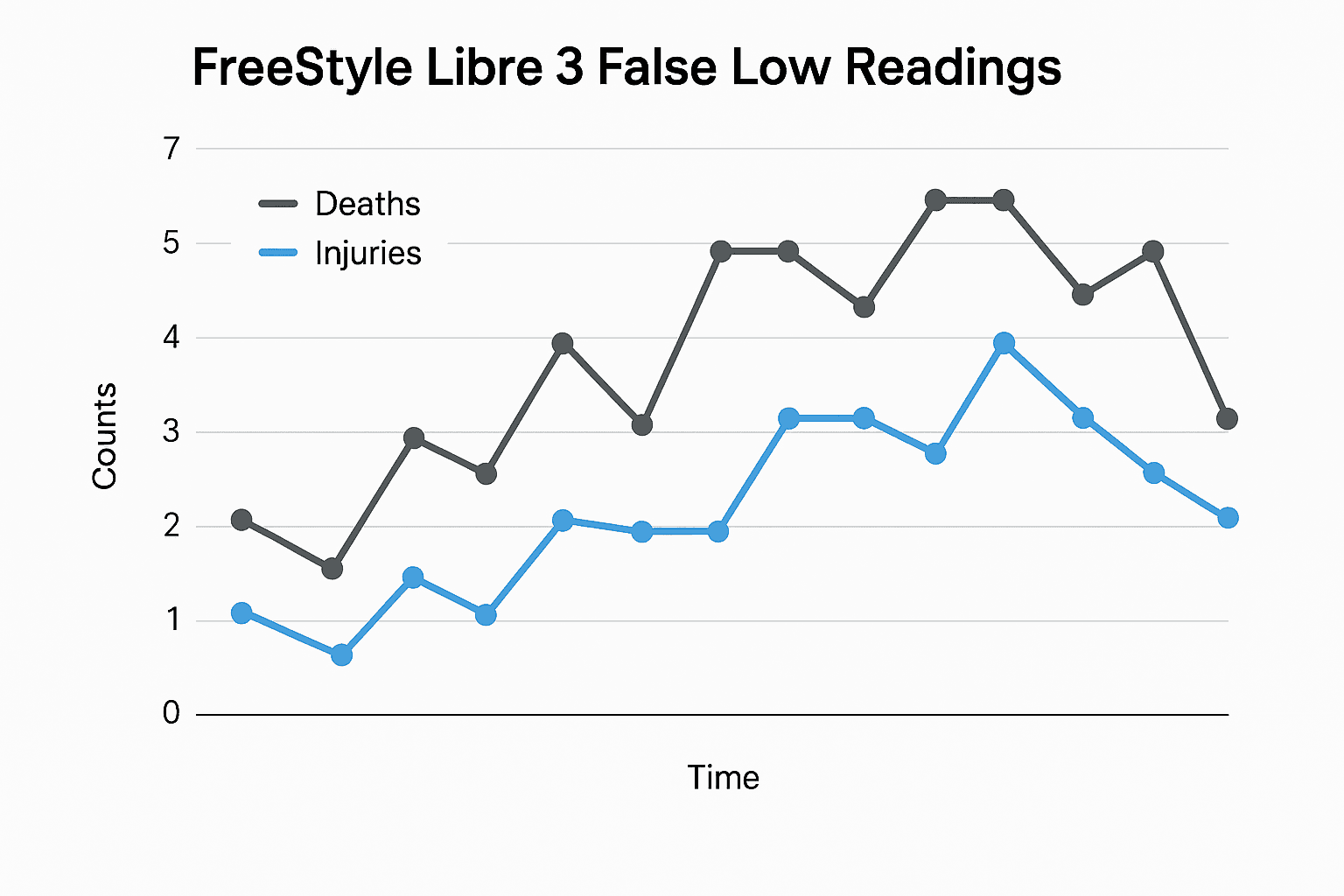
Public health officials and insurers will be pressured to act quickly to prevent care interruptions, ensure coverage for replacement devices and test supplies, and to support outreach to people who may not receive manufacturer communications promptly. Community health centers, diabetes educators and safety net clinics will play a crucial role in checking patients’ devices and distributing backup supplies.
For patients, the immediate priorities are clear. Stop using any sensor from an affected lot, verify lot numbers as Abbott has instructed, and use fingerstick testing when readings seem inconsistent with symptoms. For policymakers and health systems, the incident is a reminder that the growing reliance on medical devices demands robust surveillance, rapid corrective action, and deliberate measures to keep the most marginalized patients safe.


